Particle size determination Andersen
Andersen Evaluation Kit
Manual test apparatus is available for evaluation and method development on a commercial loan or purchase basis. The apparatus uses the core technology at the heart of the Xelize range. Methods developed using the manual apparatus can be transferred onto platforms in the Xelize range.
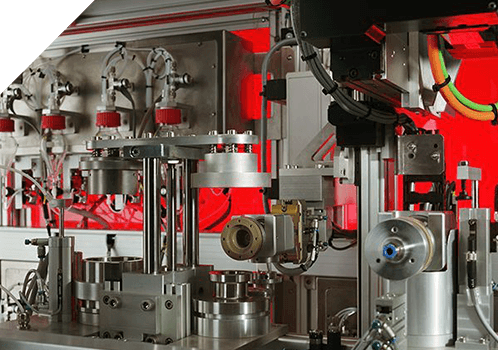
Key Benefits of Xelize® Automation
Reduced variability in results
Increased volumes of test data
Increased productivity
Greatly reduced analyst time spent on labour intensive processes
Significant reduction in Health and Safety issues including WR-ULD
Contained handling of drug product, minimising operator exposure
Upgrade paths throughout the Xelize range
Harmonised testing throughout the Xelize range
Payback typically achieved within 12 months of purchase
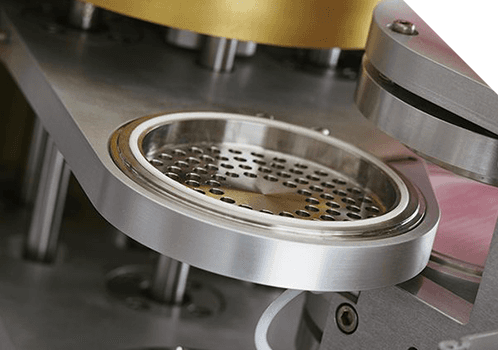
Xelize®
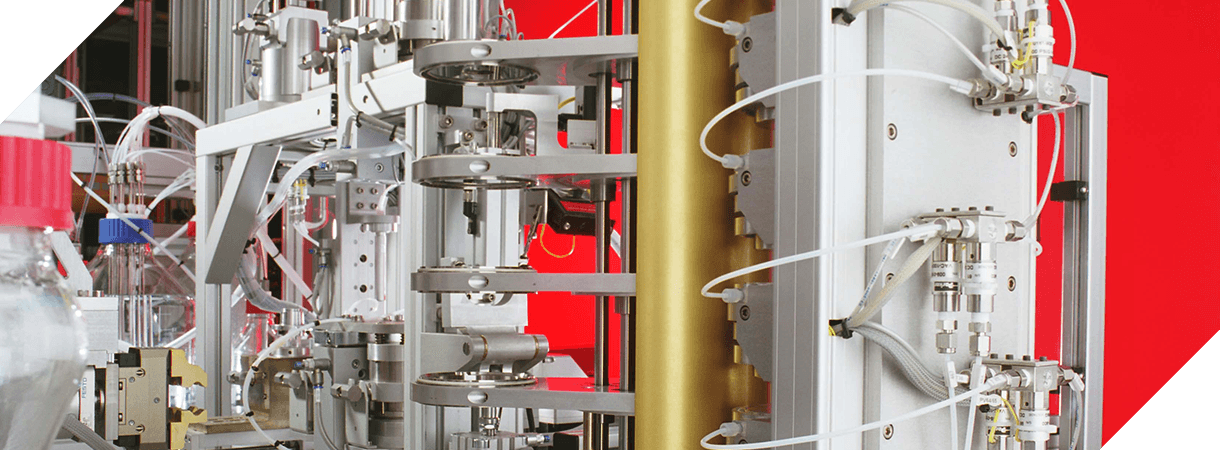
1 Series The Xelize® 1 Series are analyst workstations. They automate individual stages of the particle size determination process. 3 Series The Xelize® 3 Series are semi automated platforms. They automate the entire particle size determination process, including waste firing, dose collection, dose recovery, assay preparation, clean up and plate coating. 5 Series The Xelize® 5 Series are fully automated systems. They offer 24/7 high throughput unattended operation.

Get in contact with us
![[object Object]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2Fpk0repno%2Fproduction%2Fdbf3a5f83007da9e59edb68fb82b530f003f1265-376x220.png&w=828&q=75)
![[object Object]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2Fpk0repno%2Fproduction%2F1129b34fb570be1e0a58285546ffbcba387248c2-838x342.png&w=1920&q=75)